Welcome to the realm of chemical formula writing, where the criss cross method reigns supreme! This comprehensive guide, entitled “Writing Chemical Formulas Criss Cross Method Worksheet Answers,” embarks on a journey to unravel the intricacies of this technique, empowering you to conquer the challenges of formula writing with precision and confidence.
Delving into the heart of the criss cross method, we will meticulously dissect its steps, unraveling the secrets behind its effectiveness. Through a series of illustrative examples, we will witness the method’s prowess in crafting formulas for a diverse array of compounds, solidifying your understanding of its practical applications.
1. Understanding the Criss Cross Method
The criss cross method, also known as the exchange method, is a simple and systematic approach for writing chemical formulas for ionic compounds. It involves exchanging the charges of the ions involved to ensure that the overall charge of the compound is neutral.
To use the criss cross method, follow these steps:
- Write the symbols of the cation (positive ion) and anion (negative ion) involved.
- Criss cross the charges of the ions, making sure to swap the signs (+ and
).
- Write the subscripts of the ions based on the criss crossed charges. If the charges are the same, no subscript is needed.
- Simplify the subscripts, if possible, to obtain the lowest whole number ratio.
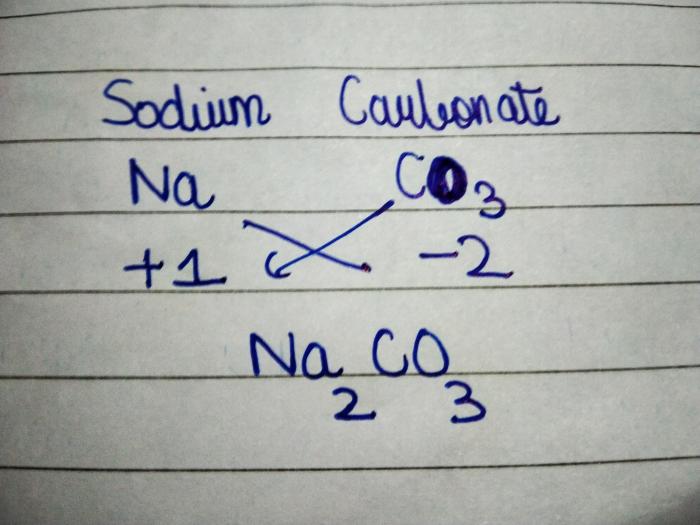
For example, to write the chemical formula for sodium chloride (NaCl), the steps are:
- Na +and Cl –
- Na –Cl +
- NaCl
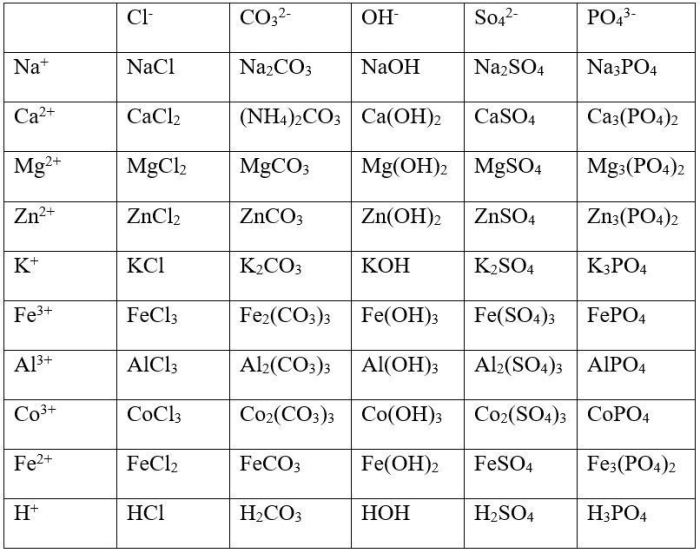
2. Worksheet Answers, Writing chemical formulas criss cross method worksheet answers
| Formula | Name |
|---|---|
| NaCl | Sodium chloride |
| CaO | Calcium oxide |
| Fe2O3 | Iron(III) oxide |
| CuSO4 | Copper(II) sulfate |
| Al2(SO4)3 | Aluminum sulfate |
3. Advantages and Disadvantages
Advantages of the Criss Cross Method:
- Simple and easy to use, especially for beginners.
- Provides a systematic approach for writing chemical formulas.
- Ensures that the overall charge of the compound is neutral.
Disadvantages of the Criss Cross Method:
- May not be suitable for complex ions or compounds with variable oxidation states.
- Can lead to incorrect formulas if the charges of the ions are not correctly determined.
- Does not provide information about the structure or bonding of the compound.
4. Variations and Extensions
Variations of the criss cross method include:
- Stock method:Uses Roman numerals to indicate the oxidation state of the metal ion.
- Prefix method:Uses prefixes like mono-, di-, tri-, etc. to indicate the number of atoms of each element.
Extensions of the criss cross method can be used to write formulas for more complex compounds, such as:
- Polyatomic ions:Ions that contain more than one atom, such as sulfate (SO 42-) or nitrate (NO 3–).
- Hydrates:Compounds that contain water molecules, such as copper(II) sulfate pentahydrate (CuSO 4·5H 2O).
5. Tips and Tricks
- Make sure to correctly determine the charges of the ions involved.
- Simplify the subscripts to obtain the lowest whole number ratio.
- Check the overall charge of the compound to ensure that it is neutral.
- Practice writing chemical formulas using the criss cross method to improve your skills.
- Refer to a periodic table or other resources for information on the charges of ions.
Common Queries: Writing Chemical Formulas Criss Cross Method Worksheet Answers
What is the criss cross method?
The criss cross method is a technique used to write balanced chemical formulas by exchanging the charges of the ions involved.
How do I use the criss cross method?
To use the criss cross method, first write the symbols of the ions involved. Then, criss cross their charges and simplify the subscripts.
What are the advantages of using the criss cross method?
The criss cross method is a simple and effective way to write balanced chemical formulas. It is especially useful for writing formulas for ionic compounds.