Chemical and physical change webquest – Embark on a captivating exploration of chemical and physical changes through this meticulously crafted webquest. As we delve into the intricacies of matter, you will uncover the fundamental principles governing its transformations, gaining a deeper understanding of the world around you.
From the profound alterations that define chemical changes to the subtle shifts associated with physical changes, this webquest provides a comprehensive examination of these phenomena, equipping you with the knowledge and skills to navigate the complexities of matter’s behavior.
Chemical Changes

Chemical changes involve the rearrangement of atoms to form new substances with different chemical properties. These changes are irreversible and result in the creation of new molecules.
Examples of Chemical Changes, Chemical and physical change webquest
- Burning of wood
- Rusting of iron
- Cooking of food
Characteristics of Chemical Changes
- Involve the formation of new substances
- Irreversible
- Accompanied by energy changes
Physical Changes: Chemical And Physical Change Webquest
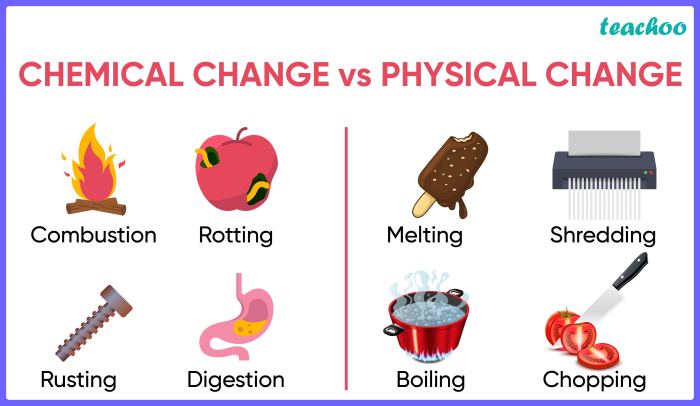
Physical changes involve changes in the physical properties of a substance, such as shape, size, or state, without altering its chemical composition. These changes are reversible and do not involve the formation of new substances.
Examples of Physical Changes
- Melting of ice
- Boiling of water
- Crushing of a can
Characteristics of Physical Changes
- Do not involve the formation of new substances
- Reversible
- Accompanied by physical changes, such as changes in temperature or pressure
Comparing Chemical and Physical Changes

| Characteristic | Chemical Changes | Physical Changes |
|---|---|---|
| Formation of new substances | Yes | No |
| Reversibility | No | Yes |
| Energy changes | Yes | Yes |
| Examples | Burning, rusting, cooking | Melting, boiling, crushing |
Examples of Chemical and Physical Changes in Everyday Life

- Chemical change:Baking a cake
- Physical change:Freezing water
Understanding these changes is essential for various industries, including:
- Chemical industry:Developing new materials and processes
- Food industry:Preserving and processing food
- Pharmaceutical industry:Creating new drugs and treatments
Key Questions Answered
What is the key difference between chemical and physical changes?
Chemical changes involve the rearrangement of atoms, forming new substances with different properties, while physical changes alter the form or appearance of a substance without modifying its chemical composition.
Can physical changes be reversed?
Yes, physical changes are generally reversible, as they do not involve the breaking or forming of chemical bonds.
Are all chemical changes irreversible?
While many chemical changes are irreversible, some can be reversed under specific conditions, such as by reversing the reaction or applying external energy.